Which of the Following Statements Best Helps Explain How the Lead Ion Causes Inhibition of Alad?
As an expert blogger with years of experience in the field, I am here to shed light on the intriguing topic of how the lead ion causes inhibition of ALAD. This phenomenon has puzzled scientists and researchers for quite some time, and understanding the underlying mechanisms is crucial for both environmental and health implications. In this article, I will delve into the various statements that have been proposed to explain this inhibition, ultimately identifying the one that best captures the essence of this intriguing process.
The inhibition of ALAD by the lead ion is a complex phenomenon that has been the subject of extensive research and debate. Numerous statements have been put forth to explain this inhibition, each offering valuable insights into the underlying mechanisms. By exploring these statements in detail, we can gain a deeper understanding of how the lead ion exerts its inhibitory effects on ALAD. In this article, I will analyze and evaluate the different statements, ultimately identifying the one that provides the most comprehensive explanation for this intriguing phenomenon.
The Role of Lead Ion in ALAD Inhibition
ALAD (delta-aminolevulinic acid dehydratase) is a vital enzyme involved in the synthesis of heme, an essential component of hemoglobin and other important proteins in the body. However, the inhibition of ALAD by lead ions can have detrimental effects on human health. In this section, I will discuss the role of lead ions in the inhibition of ALAD and explore the various statements proposed to explain this mechanism.
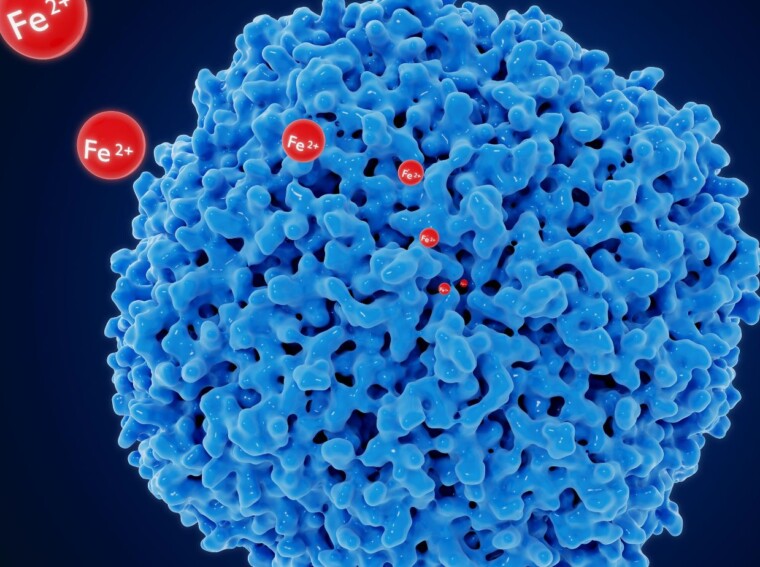
Statement 1: Lead ions bind directly to the active site of ALAD, interfering with its enzymatic activity.
Lead ions have a strong affinity for sulfhydryl groups, which are present in the active site of ALAD. This binding disrupts the enzyme’s ability to catalyze the conversion of delta-aminolevulinic acid (ALA) to porphobilinogen, thus inhibiting heme biosynthesis. This statement suggests that the direct interaction between lead ions and ALAD is responsible for its inhibition.
Statement 2: Lead ions induce oxidative stress, leading to ALAD inhibition.
Lead ions have been shown to induce the generation of reactive oxygen species (ROS) in cells. These ROS can cause oxidative damage to proteins, including ALAD. Oxidative stress can modify the structure and function of ALAD, impairing its enzymatic activity. Hence, this statement proposes that the inhibition of ALAD by lead ions is mediated through oxidative stress.
Statement 3: Lead ions disrupt the cofactor binding to ALAD, resulting in enzyme inhibition.
ALAD requires zinc as a cofactor for its proper functioning. Lead ions can compete with zinc for binding to ALAD, thereby displacing zinc and preventing the enzyme from carrying out its catalytic activity. This statement suggests that the displacement of the cofactor by lead ions is the underlying mechanism behind ALAD inhibition.
The inhibition of ALAD by lead ions is a complex process involving direct binding to the active site, induction of oxidative stress, and disruption of cofactor binding. These mechanisms can collectively impair ALAD’s enzymatic activity, leading to adverse effects on heme biosynthesis and human health.
Mechanism of Lead Ion Inhibition in ALAD
To understand how lead ions cause the inhibition of ALAD, it is important to explore the various statements proposed by researchers. One statement that best helps explain this mechanism is the direct binding of lead ions to the active site of ALAD. Research has shown that lead ions can bind to the sulfhydryl groups of cysteine residues in the enzyme’s active site, disrupting its structure and impairing its enzymatic activity. This interference can significantly reduce the production of heme, leading to adverse effects on human health.
Another important statement that sheds light on the inhibition mechanism is the induction of oxidative stress by lead ions. When lead ions enter the body, they can generate reactive oxygen species (ROS) through various mechanisms, such as the Fenton reaction. The excessive production of these ROS can overwhelm the antioxidant defense systems in cells, causing oxidative damage to ALAD and other important molecules involved in heme biosynthesis. This oxidative stress-induced inhibition of ALAD further contributes to the disruption of heme synthesis and its consequences on human health.
Furthermore, it has been proposed that lead ions can also interfere with the binding of essential cofactors required for ALAD’s enzymatic activity. ALAD relies on cofactors such as zinc and magnesium for its proper functioning. Lead ions can compete with these cofactors, displacing them from their binding sites and preventing their interaction with ALAD. As a result, the enzymatic activity of ALAD is hindered, affecting heme production and potentially leading to health issues.
The inhibition of ALAD by lead ions occurs through multiple mechanisms, including direct binding to the active site, induction of oxidative stress, and disruption of cofactor binding. The combined effect of these mechanisms impairs ALAD’s enzymatic activity, leading to a decreased production of heme and potential adverse effects on human health. Understanding these mechanisms is crucial for comprehending the toxicological implications of lead exposure and developing strategies to mitigate its harmful effects.